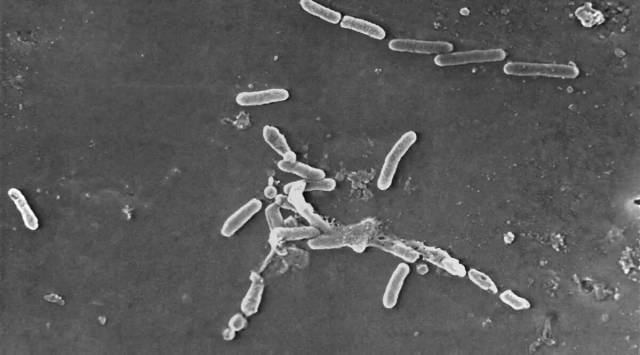
Global Pharma Healthcare, a company based in Chennai, has been instructed to stop producing all “ophthalmic preparations” after a joint inspection by central and state drug control officers found a link between its eye drops and blood-stream infections.
The company, which manufactures at least 14 eye products along with cardiovascular drugs, antibiotics, diuretics, painkillers, creams, ointments, and syrups, has also been directed to halt the manufacturing of all ophthalmic preparations until the investigation is completed.
According to the investigation report, the company exported 24 batches of the product in question in 2021 and 2022. Although the inspectors did not find any stock of the product at the company, they collected control samples and raw material samples for further analysis.
The investigation report also revealed that the company had conducted media fill validation tests biannually and yearly stability studies. A root cause analysis for the current complaint is currently underway at the company.
In addition, the company had voluntarily recalled the eye drops from the US market after the US Food and Drug Administration (FDA) initiated an investigation into a bacterial infection linked to the product. The company stated that the eye drops were distributed nationwide in the USA over the internet.
This incident follows cases of contaminated Indian-manufactured syrups linked to deaths in the Gambia and Uzbekistan, leading to the shutdown of the facilities by the Indian regulator. In both cases, the syrups allegedly contained contaminants di-ethylene glycol and ethylene glycol.
According to sources in the Union Health ministry, the eye drop in question is not sold in India.